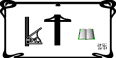
Endogenous
cannabinoids (endocannabinoids) are small molecules biosynthesized from
membrane glycerophospholipid.
The endocannabinoid system (ECS) is highly
conserved in evolution dating back to at least 600 million years.
It
consists of:
(i) lipid endocannabinoids;
(ii) their receptors
such as the Gprotein-coupled receptors, cannabinoid
receptor 1 (CB1), cannabinoid receptor 2
(CB2), and a ligand-gated cation channel vanilloidreceptor 1
(i.e., TRPV1);
(iii) the enzymes such as fatty acidamide hydrolyase
(FAAH) that regulate the levels of endocannabinoids in vivo.
The ECS
impacts several aspects of mammalianphysiology, particularly in the gut.
Endogenous cannabinoids such as
anandamide (AEA) belong to
theN-acylethanolamine family.
Anandamide (AEA) is an endogenous
intestinal cannabinoid that controls appetite and energy balance by engagement
of the enteric nervous system through cannabinoid receptors.
Here, we
uncover a role for AEA and its receptor, CB2, in the regulation of
immune tolerance in the gut and the pancreas.
The pungent molecule
capsaicin (CP) has a similar effect as AEA; however, CP acts by engagement of
the vanilloid receptor TRPV1, causing local production of AEA, which acts
through CB2.
We show that the engagement of the
cannabinoid/vanilloid receptors augments the number and immune suppressive
function of the regulatory CX3CR1hi macrophages (Mf), which express the highest
levels of such receptors among the gut immune cells. |
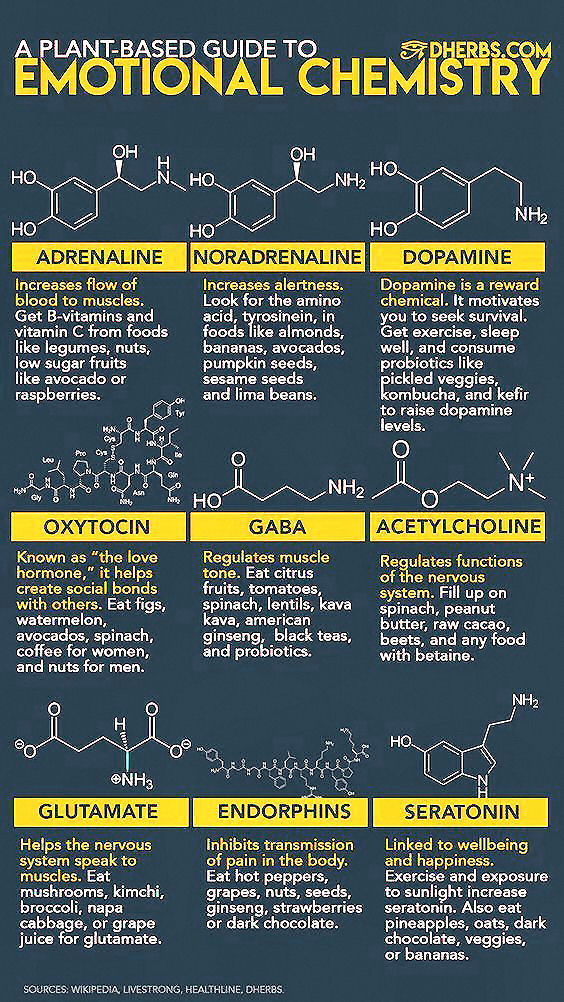
The human genome,
regardless of race, holds an informational blueprint capable of producing 17
different carbohydrate
active enzymes (CAzymes1).
These CAzymes evolved primarily to digest
terrestrial plants, and took millions of years to develop.
The
average human microbiome, on the other hand, is far more dynamic, and
contains many orders of magnitude more CAzymes than the generically shared
human genome is capable of producing itself.
One study estimated there
are about 16,000 different CAzymes in the human gut microbiome.
The
human digestive symbiont Bacteroides thetaiotaomicron3, alone, contains
261 carbohydrate digesting enzymes known as glycoside hydrolases and
polysaccharide lyases. |
gastrointestinal system
In the past centuries, different
preparations of marijuana have been used for the treatment of gastrointestinal
(GI) disorders, such as GI pain, gastroenteritis and diarrhea.
Δ9tetrahydrocannabinol, as well as endogenous and synthetic
cannabinoids, exert their biological functions on the gastrointestinal tract by
activating two types of cannabinoid receptors, CB1 receptor and
CB2 receptor.
While CB1 receptors are located in
the enteric nervous system and in sensory terminals of vagal and spinal neurons
and regulate neurotransmitter release, CB2 receptors are mostly
distributed in the immune
system, with a role presently still
difficult to establish.
Under pathophysiological conditions, the endocannabinoid system conveys
protection to the GI tract, eg from inflammation and abnormally high gastric
and enteric secretion.
Anandamide and
2-AG have been shown to inhibit the inflammatory
processes triggered upon activation of the toll-like receptor complex
CD14/TLR4/MD2 (i.e., LPS and carrageenan stimulation).
The same effect
has also been reported for other CB2 receptor agonists like
JWH133,12 HU-308,13 and N-alkylamides.
Cannabinoids have already been shown to suppress
several macrophage functions, including phagocytosis, cytolysis and
cytokine
secretion.
Identification of the mRNA for the CB1
receptor in human neuroblastoma SH-SY5Y cells, and the mRNA and protein for the
CB2 receptor in human microglia and THP-1 cells.
THP-1 cells
appear generally to be a good model for human macrophages having a range of
properties similar to microglia and other mononuclear phagocytes, including
release of such products as superoxide anion, TNF-α, IL-1ß,
prostaglandin E2.
Conclusion: The endogenous cannabinoid system is
physiologically involved in the protection against excessive inflammation in
the colon, both by dampening smooth muscular irritation caused by inflammation
and by controlling cellular pathways leading to inflammatory responses. These
results strongly suggest that modulation of the physiological activity of the
endogenous cannabinoid system during colonic inflammation might be a promising
therapeutic tool for the treatment of several diseases characterized by
inflammation of the gastrointestinal tract.
Conclusion: Pharmacological eg●
endocannabinoid levels may be a promising strategy to counteract intestinal
inflammation and colon
cancer.
The two forms of IBD,
ulcerative
colitis (UC) and Crohn's disease (CD) have rapidly increased in the past
years in Western countries ranging at a prevalence of more than 200 cases per
100,000 inhabitants.
Initially, restitution is achieved by epithelial
dedifferentiation and migration, followed by proliferation, and, finally,
differentiation and
maturation. LPA has been shown to enhance intestinal epithelial wound
healing through increased epithelial cell migration.
gastric cancer
Conclusion: Cannabinoids as a new gastric cancer
therapy.
Conclusion: Cannabinoids as a good palliative agent
for cancer patients receiving paclitaxel.
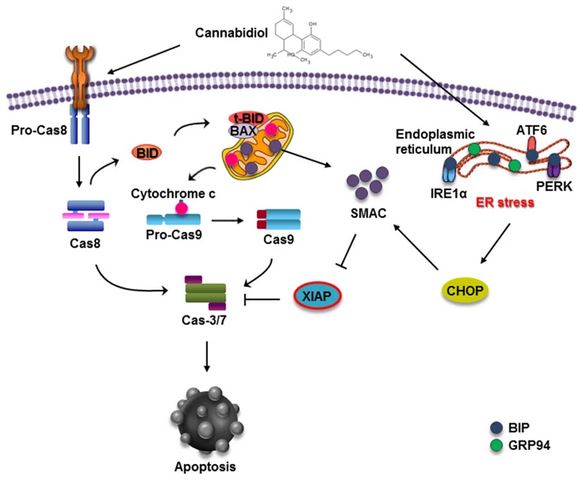
Conclusion: Our study showed that CBD
induces apoptotic cell death in gastric
cancer cells, which is triggered by ER stress
generation and subsequent XIAP inhibition by Smac. Taken together, our
results suggest the potential of CBD in novel treatments against gastric
cancer.
To study the effect of CBD on gastric cancer in
vitro, we treated the gastric cancer SGC-7901 cells with different
concentrations of CBD for 24 and 48 h. These results indicated that CBD could
effectively induce cell cycle arrest at the G0–G1 phase by inhibiting CDK2
and cyclin E expression. We found that as the concentration of CBD increased,
the percentage of apoptotic cells in the SGC-7901 population increased. These
results indicated that CBD effectively induced apoptosis in SGC-7901 cells.
chronic liver disease
Conclusion: The EC system is strongly up-regulated
during chronic liver diseases. Until now it has been implicated in the
pathogenesis of fatty liver
disease associated with obesity,
alcohol abuse, and
hepatitis C, in the progression of fibrosis to cirrhosis, and in the
development of portal
hypertension, hyperdynamic circulatory syndrome and its complications, and
cirrhotic cardiomyopathy.
Furthermore, the EC system can participate in the pathogenesis of acute liver
injury by modulating the mechanisms responsible for cell injury and
inflammatory response. Thus, targeting the CB1 and CB2
receptors represents a potential therapeutic goal for the treatment of liver
diseases.
Conclusion: Endocannabinoid-based therapies,
combining CB2 agonists and CB1 antagonists may open novel
therapeutic perspectives for the treatment of chronic liver diseases.
Conclusion: CB1 receptors have been
implicated in the pathogenesis of several lesions such as liver fibrogenesis,
alcoholic and metabolic steatosis, or circulatory failure associated with
cirrhosis. In contrast, stimulation of hepatic CB2 receptors is
emerging as an overall protective pathway with antifibrogenic properties and
beneficial effects on liver inflammation, alcoholic fatty liver and hepatocyte
survival and regeneration.
The present study shows that activation of
CB2 receptors alleviates CCl4-induced hepatitis and accelerates
liver regeneration, therefore identifying CB2 agonists as potential
beneficial hepatoprotective agents.
Conclusion: CB2 receptors
reduce liver injury and promote liver regeneration following acute insult, via
distinct paracrine mechanisms involving hepatic myofibroblasts.
Conclusion: Our study shows that CB1
receptor antagonists hold promise for the treatment of liver fibrosis.
Conclusion: CBD can alleviate lipid accumulation in
both an in vitro HepG2 cell model and an in vivo binge alcohol treatment model
by multiple mechanisms.
Conclusion: These findings demonstrate that
CB2 receptors display beneficial effects on alcohol-induced
inflammation by regulating M1/M2 balance in Kupffer cells, thereby reducing
hepatocyte steatosis via paracrine interactions between Kupffer cells and
hepatocytes.
Conclusion: Our results suggest that THCV and CBD
might be used as new therapeutic agents for the pharmacological treatment of
obesity- and metabolic
syndrome-related NAFLD/hepatosteatosis
Results:
Δ9-THC- and
JWH-015-induced autophagy and apoptosis relies on CB2 receptor
activation
Δ9-THC and JWH-015 inhibit the growth of the
human HCC lines HepG2 and HuH-7 via autophagy stimulation
AMPK activation
and TRB3 upregulation involved Δ9-THC- and JWH-015-induced
autophagy and apoptosis of HCC cells
AMPK and TRB3 regulate
cannabinoid-induced autophagy of HCC cells through different
mechanisms
Activation of AMPK by cannabinoids relies on CAMKK
nausea
Considerable evidence demonstrates that manipulation
of the endocannabinoid system regulates nausea and vomiting in humans and other
animals. The anti-emetic effect of cannabinoids has been shown across a wide
variety of animals that are capable of vomiting in response to a toxic
challenge. CB1 agonism suppresses vomiting, which is reversed by
CB1 antagonism, and CB1 inverse agonism promotes
vomiting.
One of the oldest pharmacological remedies for
nausea and vomiting is the plant cannabis. In clinical trials, cannabis-based
medicines have been found to be effective anti-emetics and even surpass some
modern treatments in their potential to alleviate nausea.
Conclusion: Cannabinoids have great promise as
treatments for nausea and that their anti-nausea effects may be mediated by the
interoceptive insular cortex.
Conclusion: This model may be a useful tool for
elucidating the neurobiology of nausea and the role that the endocannabinoid
system plays in the regulation of this distressing condition.
Conclusion: Future efforts aimed at developing new
endocannabinoid-based anti-nausea and anti-emetic therapies are clearly
warranted.
kidney fibrosis
Conclusion: Our data suggest
a possible implication of
the endocannabinoid system in the physiology and development of the human
kidney.
Conclusion: CB1 has a major role in the
activation of myofibroblasts and may be a new target for treating
chronic kidney
disease.
In conclusion, in the present study we showed that
the CB receptor agonists AM841 and CB 13 displayed protective and therapeutic
effects on colitis in mice. Most importantly, we demonstrated that the
anti-inflammatory action of
the cannabinoids was mediated through CB1 and CB2
receptors localized centrally and possibly to a lesser extent - peripherally.
To the best of our knowledge, this is the first evidence showing the
involvement of central CB receptors in development and treatment of colitis.
These results are crucial for our understanding of the pharmacology of CB
ligands in GI inflammation and may have potential applications in the
development of future treatment strategies for IBD in humans.
Cannabidiol, a safe and non-psychotropic ingredient
of marijuana, exerts pharmacological effects (e.g., antioxidant) and mechanisms
(e.g., inhibition of endocannabinoids enzymatic degradation) potentially
beneficial for the inflamed gut.
Crohn's
The endocannabinoid system is involved in almost all
major immune events.
In murine colitis, cannabinoids decrease
histologic and microscopic inflammation.
In humans, cannabis has been
used to treat a plethora of gastrointestinal problems, including anorexia,
emesis, abdominal pain, diarrhea, and diabetic gastroparesis.
In an
observational study in 30 patients with Crohn's disease (CD), we found that
medical cannabis was associated with improvement in disease activity and
reduction in the use of other medications.
In a more recent
placebo-controlled study in 21 chronic CD patients, we showed a decrease in the
CD activity index >100 in 10 of 11 subjects on cannabis compared to 4 of 10
on placebo.
Complete remission was achieved in 5 of 11 subjects in the
cannabis group and 1 of 10 in the placebo group.
Conclusion: A short course (8 weeks) of THC-rich
cannabis produced significant clinical, steroid-free benefits to 10 of 11
patients with active Crohn's disease, compared with
placebo, without side effects.
As with the eCB system, many eCBome members regulate
several physiological processes, including energy intake and storage,
glucose and lipid
metabolism and
pancreatic
health, which contribute to the development of type 2 diabetes (T2D).
Preclinical studies increasingly support the notion that targeting the eCBome
may beneficially affect T2D. The eCBome is implicated in T2D at several levels
and in a variety of tissues, making this complex lipid signaling system a
potential source of many potential therapeutics for the treatments for T2D.
Activation of CB-1 receptors in the gastrointestinal
tract may also be relevant for the
pathogenesis of obesity. The response of circulating ghrelin to fasting was
diminished with rimonabant, suggesting that CB-1 receptors are involved in
ghrelin secretion |