|

ęther is the name of the
essence that fills the void of space (heavens)
beyond the sky (atmoshpere) in
which the planetary bodies reside.
Thought to fill all space, up until
the time Albert Einstien
came up with the Theory of Relativity, ęther allowed corpuscles (electromagnetic waves)
to pass through and interact with matter,
without exerting any
résistance.
This is the necessary explanation in the
mechanistic universe of Isaac
Newton for how light (photons) are able to
travel the immense empty distance from the
distant sun to reach the
surface of the Earth to warm it
sparking life.
Shaping principles of all
scientists at the time would have been grounded in this definition of ęther,
later reflected in the terms vacuum and void.


A
pleasant-smelling, volatile, flammable and
colorless liquid
used as an anesthetic or as a solvent in industrial
processes
composed of an oxygen atom connected to two alkyl or aryl
groups.
Simple ethers/symmetrical ethers:
diethyl ether, dimethyl ether, polybrominated diphenyl ether ...
Mixed
ethers/asymmetrical ethers:
methyl ethyl ether, methyl phenyl ether,
methyl tert-butyl ether, ...
Glycol ethers:
"E-series" = ethylene
oxide
"P-series"
= propylene oxide
E-series
glycol ethers, ethylene glycols, are found in
pharmaceuticals, sunscreens, cosmetics, inks, dyes and water-based
paints.
P-series glycol ethers, propylene
glycols, are used in degreasers, cleaners, aerosol paints and
adhesives.
P-series glycol ethers are marketed as lower toxicity than
the E-series.
Occupational exposure to glycol ethers is related to low
motile sperm count.
Ethylene glycol monomethyl ether - 2-methoxyethanol
is toxic to the bone marrow and testicles.
High level exposures at risk
for granulocytopenia, macrocytic
anemia, oligospermia,
and azoospermia.
Ethylene
glycol monobutyl ether - 100 - 500 ppm of 2-butoxyethanol can cause
adrenal tumors in
animals.
Ethylene glycol monophenyl
ether - Japan and EU consider phenoxyethanol safe for use as a preservative
with a maximum concentration of 1.0%.
The most common water-based
antifreeze solutions used in electronics and automotive cooling are mixtures of
water and either ethylene glycol (EGW) or propylene glycol
(PGW).

1846 Christian Schönbein
accidentally synthesizes guncotton.
1847
Gunpowder manufacturers John Hall & Sons began to produce
guncotton using the Schönbein process at a factory in Faversham,
Kent.
Ascanio Sobrero first synthesizes nitroglycerin.
1867 Alfred Nobel invents
dynamite, an explosive made of nitroglycerin, sorbents (diatomaceous earth or clay) and
stabilizers.
1875 Alfred Nobel
invents the first plastic explosive - gelignite.
Gelignite is an
explosive material consisting of nitrocellulose dissolved in either
nitroglycerine or nitroglycol and mixed with wood pulp and saltpetre.
1886 John Wesley Hyatt devises a
method of making billiard balls with collodion or
nitrocellulose.
Nitrocellulose is a highly flammable compound formed by
nitrating cellulose through exposure to a mixture of nitric and hydrochloric or
sulfuric
acid.
Nitrocellulose is used to make smokeless gun powder,
waterproof fuses in pyrotechnics, inks, adhesives, varnishes, resins and
lacquer coatings.
John Wesley Hyatt grinds nitrocellulose into a fine
pulp and combines it with camphor, forms it in a mold and places it under
pressure and heat.
Pressure, applied steadily over time, creates a
dense durable billiard ball.
1889
Frederick Abel and James Dewar patent a smokeless propellant
consisting of guncotton and nitroglycerine mixed together in a small amount of
petroleum jelly called cordite.
1916
Chaim Weizmann uses Clostridium acetobutylicum to ferment
starch and cellulose yielding 3 parts of acetone, 6 of butanol, and 1 of
ethanol.
ATCC 824 is named the "Weizmann Organism".

A polymer is any of a class of natural or synthetic substances
composed of very large molecules, called macromolecules, which are multiples of
simpler chemical units called monomers.
Polymers make up many of the
materials in living organisms and are the basis of many minerals and man-made
materials.
Polymers can occur organically like wool, cotton, or wood, or
they can be synthesized into semi-organic or fully synthetic materials.
Polymer fibers consist of polymer chains that have stronger molecular
bonds than elastomers.
Fibers are more rigid and less elastic than
elastomers and can be composed of both natural and synthetic materials.
Polymers classified as plastics are either thermoplastics and
thermosets.
Thermoplastics are more rigid than fibers and elastomers and
are distinguished by their ability to retain their molecular structure when
exposed to heat.
When heated to their melting point, thermoplastics
will melt rather than burn, making them ideal for shaping and
forming.
Thermoplastic solvents include methyl ethyl ketone, methyl
isobutyl ketone, methylene chloride, vinyl trichloride, ethylene dichloride,
methylene chloride, toluene, xylene, cyclohexane, tetrahydrofuran, benzene and
hexane.
Thermoplastic solvents dissolve DNA and RNA as they are
polymers.
This is believed to be the cancer induction route of
carcinogenic solvents as partially dissolved ligands create transcription
errors when folding proteins.
Thermoplastic polymers are commonly
distributed in the form of pellets, and shaped into the final product form by
melting, pressing, or injection molding.
A thermoset, is a polymer
obtained by "curing" irreversibly hardening resin or viscous liquid
prepolymer.
Curing is induced by heat or suitable radiation and may be
promoted by high pressure, or mixing with a catalyst.
Heat is often
generated by the reaction of the resin with a catalyst.
Curing results
in chemical reactions that create extensive cross-linking between polymer
chains to produce an infusible and insoluble polymer network.
Deoxyribonucleic acid is a polymer composed of nucleic acids linked
together by a sugar-organophosphate backbone.
Polymer solvents can be
endocrine disruptors causing congential deformities.
Both polymer
catalysts and solvents may be carcinogenic and induce cancer.
Propylene
Propylene
or methyl ethylene, is an unsaturated organic compound having the chemical
formula C3H6.
It has one double bond, and is the
second simplest member of the alkene class of hydrocarbons.
Propylene
glycol
Propylene glycol or methyl ethyl glycol
(C3H8O2) is a clear, colorless, slightly
syrupy synthetic liquid at room temperature used by the chemical, food, and
pharmaceutical industries:
as an antifreeze;
to absorb extra
water;
as a solvent for food colors and flavors;
as a solvent in
the paint and plastics industries;
to maintain moisture in medicines,
cosmetics, food products.
Propylene glycol is used in various edible
items such as coffee-based
drinks, liquid sweeteners, ice
cream, whipped dairy products and soda.
Propylene glycol is metabolized
in the human body into pyruvic acid, acetic acid, lactic acid, and
propionaldehyde.
Alcoholic beverages in the US may contain up to 5
percent propylene glycol.
Certain formulations of
artificial tears,
Systane, use proplyene glycol.

Polyethylene
Polyethylene,
(C2H4)n the most common plastic, consists of a
long chain of carbon atoms with
two hydrogen
atoms attached to each carbon atom.
Uses incluse cosmetics, grocery
bags, shampoo bottles, children's toys, and bullet proof vests.
Polyethylene
glycol
C2nH4n+2On+1
Polyethylene glycol
(PEG) is a petroleum-derivative compound that is made from
ethylene glycol (ethane-1,2-diol), the main
ingredient in antifreeze.
Polyethylene
Glycol
PEG
Compounds and their contaminants
MiraLax Lawsuit
In 2008, FDA tested 8 batches of
Miralax and found small amounts of the
car antifreeze ingredients
ethylene glycol (EG) and diethylene glycol (DEG) in all
batches.
These were impurities from the manufacturing process.
The tests were performed because many of the
adverse reactions
reported were consistent with ethylene glycol poisoning.
PEGylation of Biopharmaceuticals
Polyethylene Glycol (PEG) and PEGylation of
Proteins
From Synthesis to Characterization of Site-Selective PEGylated
Proteins
Evaluation of PEGylated Protein Safety in the Absence of
Definitive Metabolism Studies
Polypropylene
Polypropylene
(C3H6)n is a thermoplastic "addition polymer" made from the combination of
propylene monomers.
As polypropylene, compatible with most
existing processing techniques,
has many commercial uses, including packaging, automotive products, housewares,
medicine, tubing, and food.
Polypropylene has better transparency than
other polyolefins.
Polypropylene has a lower specific weight, so that
lighter products are obtained in its applications.
Polyvinyl
chloride
Polyvinyl chloride
(C2H3Cl)n[2], sythesized from
vinyl chloride, is the
world's third-most widely produced polymer, after polyethylene and
polypropylene.
Polyvinyl chloride, the only plastic made with chlorine,
is 57% chlorine.
Polyvinyl
chloride requires toxic additives, including heavy metals such as
lead,
endocrine-disrupting phthalates, and
flame retardants, in order to be made
into stable and usable consumer products.
These additives are released
during both the use and disposal of PVC.
One byproduct during decomposition is
dioxin.
High levels of the
carcinogen vinyl chloride, a
toxic colorless gas with a sweet odor used to make polyvinyl chloride, are
found in 1997, 1998 and 2001.
EPA concludes cleanup of up to 355,000
contaminated sites nationwide will cost up to $280 billion over the next 35
years.
Nylon
Nylon
monomers are manufactured by a variety of routes, starting in most cases from
crude oil.
Crude oil » benzene » cyclohexane »
cyclohexanone » cyclohexanone oxime » caprolactam
»
Heat caprolactam to about 500° Farenheit in an inert
atmosphere of nitrogen
for 4-5 hours, the ring breaks and caprolactam undergoes
polymerization.
The molten mass is
passed through spinnerets to form fibres of nylon 6.
In water,
caprolactam [(CH2)5C(O)NH] hydrolyzes to
aminocaproic acid, which is used
medicinally.

Melamine
Melamine, C3H6N6, contains 67%
nitrogen by mass, and its derivatives have fire retardant properties due to its
release of nitrogen gas when burned.
Kjeldahl and Dumas
estimate protein
levels by measuring nitrogen content, so they can be misled by adding
nitrogen-rich compounds such as melamine.
World Health Organization
food safety director 0.2 mg per kg of body mass.
Butylene
Butylene, 2-methylpropene, is a series of alkenes with the
general formula C4H8.
Butene is therefore obtained
by catalytic cracking of long-chain hydrocarbons left during refining of crude
oil.
Cracking produces a mixture of products, and the butene is
extracted from this by fractional distillation.
Butene reacts with
methanol and
ethanol in the
manufacture of the gasoline oxygenate methyl tert-butyl ether
(MTBE).
Butylene
glycol
Butylene glycol, C4H10O2,
is a solvent and viscosity conditioning agent.
Butylene glycol is
commonly used as a solvent for food flavouring agents and in the manufacturing
of resins.
In biology, butylene glycol is used as a hypoglycaemic
agent.
Glycolate
Glycolic acid,
C2H4O3, is the smallest α-hydroxy
acid.
α-hydroxy acids are a group of organic carboxylic compounds
most commonly used in cosmetic applications.
α-hydroxy acids may
be derived from food products on the microscale:
glycolic acid (from sugar
cane);
lactic acid (from sour milk);
malic acid (from
apples);
citric acid (from citrus fruits);
tartaric acid (from
grape wine).
Glycolic acid is a colorless, odorless, highly soluble
hygroscopic crystalline solid.
Glycolic acid uses the hydroxyl and
carboxylic acid groups to form five member ring complexes (chelates) with
polyvalent metals.
This complexing ability is
useful in dissolution of
hard-water scale and prevention of deposition, especially in cleaning
applications.
Glycolic acid readily forms salts with active metals,
metal oxides and bases.
Glycolic acid is used in the
textile industry as
a dyeing and tanning agent, in food processing as a flavoring agent and as a
preservative.
Glycolic acid is
often included into emulsion polymers, solvents and additives for
ink and paint in order to improve flow properties
and impart gloss.
Although glycolic acid occurs naturally as a trace
component in sugarcane, beets, grapes and fruits,
DuPont synthesizes the
product through a sustainable manufacturing process in Belle, West
Virginia.
DuPont has been
the leading supplier of Glycolic acid to customers and distributors worldwide
for more than 50 years
Ethylene glycol
Ethylene glycol, (CH2OH)2, is used as
a raw material in the manufacture of polyester fibers and for antifreeze
formulations.
Ethylene glycol is an odorless, colorless, sweet-tasting,
viscous liquid.
Ethylene glycol is highly toxic.

"Carbon building
blocks of a similar size - rings or not - when polymerized (strung together in
repeating units), form plastics.
If the strung-together chains zig-zag
repeatedly instead of being straight and taught, the
polymer is potentially elastic
and might be a synthetic rubber.
Polymerization done in a factory
results in a plastic or synthetic rubber.
If you do the
polymerization, the product is a varnish
(paint) or glue.
The polymerization occurs with oxygen and/or UV
bombardment allowing the varnished, painted or glued item to "dry" or harden
into a polymer.
For varnish or pigmented varnish ("paint"), solvents
are added to retard polymerization, so in a sense the varnish or paint really
is "drying", but the real issue (goal) here is polymerization not solvent loss.
If the air's oxygen isn't enough, a chemical oxidizer is used.
"MEKP" is popular (methyl ethyl ketone peroxide). " - JI Nelson
6-carbon rings of
benzene are easily added as half the carbon-to-carbon bonds are double bonds
and can be "opened".
One of the original chemical bonds continues
to hold the ring together, while the chemical engineer attaches something new
to the other.
Plastics (whether based on rings or not) can use opened
double bonds to link the long polymerized chains to one another.
This
hardens the plastic transforming it from a bendable into a stiff polymer.
Hardening synthetic rubber by cross-linking is called "vulcanization".
Hardened oils are
margarine.
There are no calories if the double bonds of "saturated
fats" are all used up.
Body building blocks are better if they have more
than a single double bond of "polyunsaturated" to metabolize or more easily
attach another molecule, rather than
leaving the
vulcanized oils to pile up as arterial deposits.
2008 Strain of Escherichia coli is
genetically engineered to synthesize butanol with genes derived from
Clostridium acetobutylicum.
2013
First microbial production of short-chain alkanes reported - a
considerable step toward the sythetic biolgocal production of gasoline.
Fatty acyl-CoA reductase came from Clostridium
acetobutylicum.
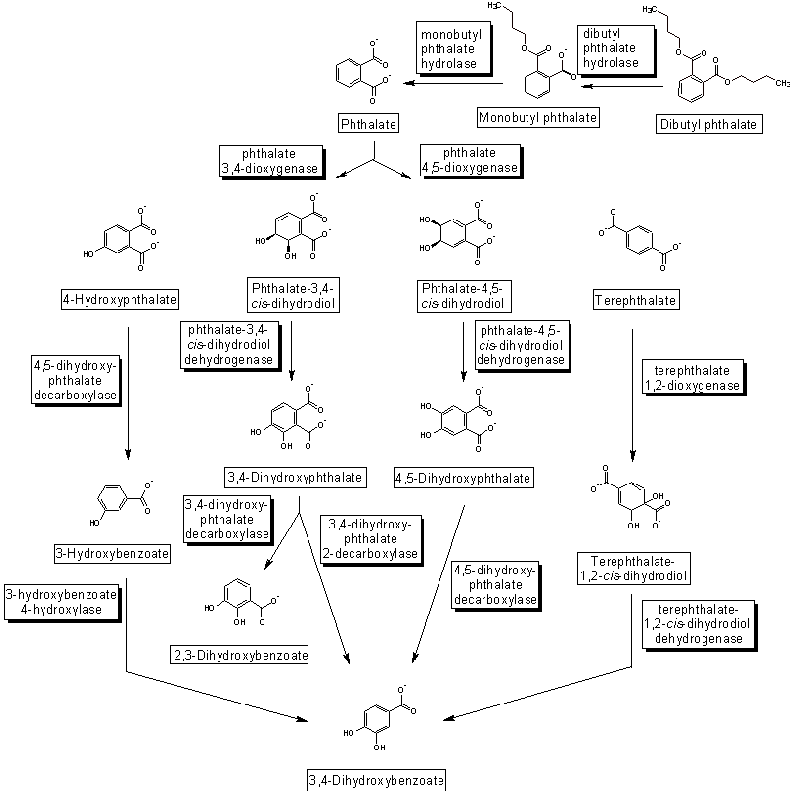
phthalates

1990s Toxicologists recognize pesticides and many
industrial compounds, including phthalates, can mimic estrogen or block
testosterone, the female and male sex hormones that
control reproductive
development.
Review of sperm counts in developed nations showed a
substantial decline since World War II, when many synthetic pesticides and
industrial compounds were introduced into the environment.
Previous
studies of men have linked phthalates to low sperm quality.
Nearly
everyone in a 1999-2000 survey of 2,500 people throughout America had
phthalates in
their urine.

2005
Phthalates widely used in plastics and
beauty products have
contaminated humans altering
the reproductive organs of baby boys.
Observers find a strong
correlation between phthalate contamination and changes in the size and anatomy
of the children's genitals.
Mothers with the
highest levels of chemical in
their urine late in their pregnancies had babies with a cluster of effects.
The span between anus and penis, anogenital distance, is
short.
Infants have smaller penises, scrotums and incomplete descent of
testicles.
Phthalate levels associated with the genital changes are not
unusually high.
Reproductive
biologists say that a shorter anogenital distance is a female like effect
in animals, a telltale sign of
decreased male hormones.
It is likely that the human effects are similar
as hormones function the same in animals and humans.
Medical equipment,
baby bottle nipples, pacifiers, teething rings, hairspray, deodorants,
perfumes, vinyl products including
upholstery, packaging, wall and floor covering, nail polishes and other
beauty products sold in
America contain phthalates which have been banned in the Europe and
Japan.
"America has become a dumping ground for
chemical filled toys that are banned in
much ofthe global industrialized world." - Fiona Ma
Europe banned 2
phthalates found in cosmetics and 6 phthalates in toys.
Japan, Mexico
and Canada have banned phthalates.
America is one of a few countries in
which phthalates are still used.


In Victorian England and the post-Civil War era in America, the
use of heavy metals in cosmetics - such as
mercury,
arsenic and
lead was
widespread.
Women and men of the 1800s/early 1900s used toxic
metals.
Toxic ingredients such as phthalates,
parabens, asbestos
talcum powder, nanoparticles,
formaldehyde, lead
acetate, coal tar, octyl methoxycinnamate
and 4-methybenxylidene camphor, triclosan, resorcinol, toulene, butylated
hydroxyanisole (BHA) are now present in cosmetics and beauty
products.
Toxic
Triclosan Banned From Soap but Lingers in Consumer
Products
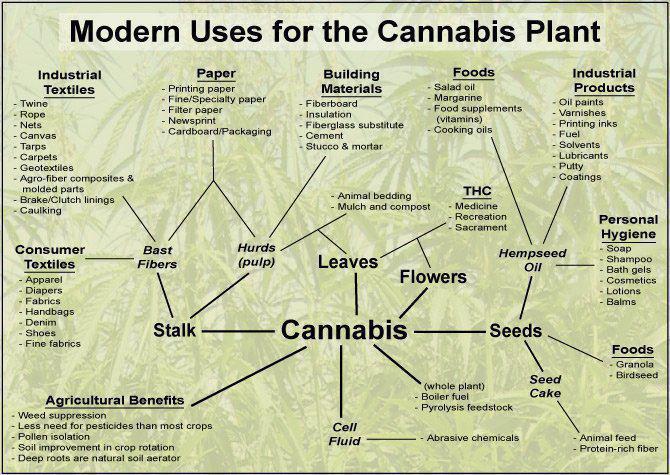
The benefits of hemp
include:
Hemp plastics are
biodegradable.
Hemp has multiple healing
properties.
Hemp clothing is extremely strong and
durable.
Hemp books last for centuries rather than decades.
Hemp
paper does not turn yellow and is very durable.
Hemp is a higher
quality fiber than wood fiber or cotton.
Hemp grows quickly to maturity
in a season where trees take a lifetime.
Hemp Paper requires far fewer caustic chemicals than paper from
trees.
Hemp seed contains one of the
highest sources of
protein in
nature as well as two essential
fatty acids, not found
anywhere else in nature, that clean your brain of amyloidal
plaques and regenerate neurons. |
|
 |
This web site is not a commercial web site and
is presented for educational purposes only.

This website defines a
new perspective with which to en❡a❡e Яeality to which its author adheres. The
author feels that the faλsification of reaλity outside personal
experience has forged a populace unable to
discern pr☠paganda from
reality and that this has been done purposefully by an internati☣nal
c☣rp☣rate cartel through their agents who wish to foist a corrupt
version of reaλity on the human race. Religi☯us int☯lerance
☯ccurs when any group refuses to tolerate religi☯us practices,
religi☸us beliefs or persons due to their religious ideology. This web
site marks the founding of a system of philºsºphy nªmed The
Mŷsterŷ of the Lumière Infinie - a ra☨ional
gnos☨ic mys☨ery re☦igion based on reaso🐍 which
requires no leap of faith, accepts no tithes, has no supreme leader, no church
buildings and in which each and every individual is encouraged to develop a
pers∞nal relati∞n with Æon and Sustainer through the pursuit
of the knowλedge of reaλity in the hope of curing the spiritual
c✡rrupti✡n that has enveloped the human spirit. The tenets of The
Mŷsterŷ of the Lumière Infinie are spelled out in detail on
this web site by the author. Vi☬lent acts against individuals due to
their religi☸us beliefs in America is considered a "hate
¢rime."
This web site in no way c☬nd☬nes vi☬lence. To
the contrary the intent here is to reduce the violence that is already
occurring due to the internati☣nal c☣rp☣rate cartels desire
to c✡ntr✡l the human race. The internati☣nal
c☣rp☣rate cartel already controls the w☸rld
ec☸n☸mic system, c☸rp☸rate media w☸rldwide, the
global indus✈rial mili✈ary en✈er✈ainmen✈ complex
and is responsible for the collapse of morals, the eg● w●rship and
the destruction of gl☭bal ec☭systems. Civilization is based on
coöperation. Coöperation with bi☣hazards at the
point of a gun.
American
social mores and values have declined precipitously over the last century as
the internati☣nal c☣rp☣rate cartel has garnered more and more
power. This power rests in the ability to deceive the p☠pulace in general
through c✡rp✡rate media by press☟ng em☠ti☠nal
butt☠ns which have been πreπrogrammed into the
πoπulation through prior mass media psychological operations. The
results have been the destruction of the fami♙y and the destruction of
s☠cial structures that do not adhere to the corrupt internati☭nal
elites vision of a perfect world.
Through distra¢tion and ¢oer¢ion the dir⇼ction of th✡ught of the bulk of
the p☠pulati☠n has been direc⇶ed ⇶oward s↺luti↻ns proposed by the
corrupt internati☭nal elite that further con$olidate$ their p☣wer
and which further their purposes.
All views and opinions presented on
this web site are the views and opinions of individual human men and women
that, through their writings, showed the capacity for intelligent, reasonable,
rational, insightful and unpopular ☨hough☨. All factual information presented
on this web site is believed to be true and accurate and is presented as
originally presented in print media which may or may not have originally
presented the facts truthfully.
Øpinion and ☨hough☨s
have been adapted, edited, corrected, redacted, combined, added to, re-edited
and re-corrected as nearly all opinion and ☨hough☨ has been throughout time but
has been done so in the spirit of the original writer with the intent of making
his or her ☨hough☨s and opinions clearer and relevant to the reader in the
present time.
Fair Use Notice

This site may contain
copyrighted material the use of which has not always been specifically
authorized by the copyright owner. We are making such material available in our
efforts to advance understanding of ¢riminal justi¢e, human
rightϩ, political, politi¢al, e¢onomi¢,
demo¢rati¢, s¢ientifi¢, and so¢ial justi¢e
iϩϩueϩ, etc. We believe this constitutes a 'fair use' of any
such copyrighted material as provided for in section 107 of the US Copyright
Law. In accordance with Title 17 U.S.C. Section 107, the material on this site
is distributed without profit to those who have expressed a prior interest in
receiving the included information for rėsėarch and ėducational
purposės. For more information see:
www.law.cornell.edu/uscode/17/107.shtml. If you wish to use copyrighted
material from this site for purposes of your own that go beyond 'fair use', you
must obtain permission from the copyright owner. |
 Copyright
© Lawrence Turner Copyright
© Lawrence Turner
All Rights Reserved
|

