"The common feature of
Acute Respiratory Distress Syndrome (ARDS) includes
systemic
hyperactivation of immune response leading to inflammation in the lungs
followed by the development of
pulmonary edema,
alveolar damage, and respiratory failure.
There are over 200,000 people
affected by ARDS annually in the US and three million people globally, and ARDS
causes over 75,000 deaths in the US alone.
ARDS can result from a wide
range of insults, and the precise nature of antigens or factors that
trigger hyperactivation of the immune
response, is unclear.
The most challenging question which remains
unanswered is whether hyperactivation of immune response seen in patients with
the severe form of COVID-19 results from
hyperimmune response against
the virus or against secondary infections seen in
these patients or a combination of both.
It is less likely that the
hyperactivation of the
immune response is against the virus itself because these are the same
patients who are immunocompromized that fail to
exhibit an optimum response to
the virus.
The prevalence of coinfection varies but it can account
for up to 50% among patients who die from COVID-19 (Lai et al., 2020).
The copathogens include, Streptococcus pneumoniae,
Staphylococcus aureus, Klebsiella pneumoniae, and the like (Lai
et al., 2020).
Some of these bacteria
produce toxins such as Staphylococcus enterotoxin B (SEB) which can
activate a large proportion of T lymphocytes, thereby causing
cytokine
storm, ARDS, and multiorgan failure." |
A
transcriptomic analysis of immune
cells from the lungs revealed an increase in mitochondrial respiratory
chain enzymes following THC treatment.
Metabolomic analysis revealed
elevated serum concentrations of amino acids, lysine, n-acetyl methionine,
carnitine, and propionyl L-carnitine in THC-treated mice.
THC caused
the downregulation of miR-185, which correlated with an increase in the
pro-apoptotic gene targets.
Interestingly, the
gene expression
datasets from the bronchoalveolar lavage fluid (BALF) of human COVID-19
patients showed some similarities between cytokine and apoptotic genes with
SEB-induced ARDS.
Collectively, this study suggests that the activation
of cannabinoid receptors may serve as a therapeutic modality to treat ARDS
associated with COVID-19. |
Tolerance is a fundamental property of the immune
system.
Tolerance involves non-self discrimination which is the
ability of the normal immune
system to recognize and respond to
foreign proteins, but not self.
Autoimmunity is invoked when
tolerance to autoantigens is broken.
Immunological tolerance within an
individual normally
begins as a fetus.
In maternal fetal tolerance T lymphocytes express
receptors for a specific antigen enters the circulation of the
developing fetus via the
placenta.
Fetal T lymphocytes orginate in the bone marrow where they
begin growth but must travel to the thymus where maturation of T lymphocytes
occurs.
Within the thymus fetal T lymphocytes encounter a variety of
antigens.
T lymphocytes, now
attenuated to
pathogens, become pathogen hunters.
Approximately 99 percent of
fetal T lymphocytes die through induction of apoptosis in the thymus attempting
to convert T lymphocytes into hunters.
An autoimmune response can
be incited through molecular mimicry.
Molecular mimicry involves the
ability of similar molecular structures from dissimilar genes or
proteins to mimic similar
organic self structures.
Dissimilar sequence partial structures may
elicite an autoimmune
response.
Virulent
proteins, through molecular surfaces,
mimic host protein
surfaces.
Through molecular mimicry a pathogen may generate natural
immunity.
The pathogen may
mimic the linear
amino acid sequence.
It may mimic the
conformational fit of the
immunodominant epitope.
In the phenomenon of immunodominance immune
responses are mounted against a few of the antigenic peptides presented on the
globular protein.
Immunodominance epitopes exist for antibody and
lymphocyte immunity.
An autoimmune response is then generated
by any similar pathogen.
Pathogens alter macrophage
function; act as mutagens to
release cytokines.
Release of cytokines spark
reformation of exosomes
altering cellular DNA creating antigen release
mechanisms to counteract foreign
proteins.
By
distorting the
configuration of the native protein, the
immune system
attacks autoantigens, resulting in an
autoimmune or
allergic
reaction.
Due to similar sequence homology in the immunodominant
epitope between the pathogen and the host, cells and tissues of the host
associated with the protein are hunted down as a result of the autoimmune
response.
Findings from biological research suggest that sustained
involvement in gratifying activities
such as the creation of works of art or walking through
Pinus sylvestris forests result in
positive immune system
responses.
Cannabinoids and the immune system
The effect of
cannabimimetic agents on the function of immune cells such as T and β
lymphocytes, large granular lymphocytes and macrophages has been extensively
studied using human and animal paradigms involving whole animal models (in
vivo) as well as tissue culture systems ( in vitro).
Cannabimimetic agents act as agonists at cannabinoid
receptors.
Cannabimimetic agents have a complex effects on immune cell
function.
Activity is mediated by cannabinoid receptors expressed on
cell subtypes.
It is likely that the cannabinoid system, along with the
other neuroimmune
systems, has a subtle but significant role in the regulation of
immunity.
Cannabinoids have been shown to act as potent
immunosuppressive and anti-inflammatory agents and have
been shown to mediate beneficial effects in a wide range of
immune-mediated diseases such as
multiple sclerosis, diabetes,
septic shock,
rheumatoid arthritis, and allergic asthma. In this
review, we will focus on apoptotic mechanisms of immunosuppression mediated by
cannabinoids on different immune cell populations and discuss how activation of
CB2 provides a novel therapeutic modality against
inflammatory and autoimmune
diseases as well as malignancies of the
immune system,
without exerting the untoward psychotropic effects.
Conclusion: The potential use of cannabinoids as a
new class of anti-inflammatory
agents against a number of
inflammatory and
autoimmune diseases that are primarily triggered by activated
T lymphocytes or other cellular
immune components.
Conclusion: The direct antitumor activity of AEA
together with the absence of negative effects on T lymphocyte
functions might provide new insights into the potential use of cannabinoid
agents in cancer immunotherapy.
Conclusion: Medicinal cannabis is an invaluable
adjunct therapy for pain relief, nausea, anorexia, and mood modification in
cancer patients and is available as cookies or cakes, as sublingual drops, as a
vaporized mist, or for smoking.
Conclusion: Sustained ceramide accumulation in tumor
cells mediates cannabinoid-induced
apoptosis, as evidenced by in vitro and in vivo studies.
Conclusion: Direct antitumor activity of endogenous
cannabinoid anandamide together with the absence of negative effects on
T lymphocyte
functions.
The phytocannabinoid (-)-cannabidiol is a partial
agonist, being approximately 40 fold more potent than (+)-cannabidiol;
abnormal-cannabidiol is a full agonist. Furthermore, the abnormal-cannabidiol
(CBD) analog
trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-methyl-1,3-benzenediol
(O-1602) inhibits migration, with an IC(50) value of 33 nM. This reported
profile of agonist efficacy and potency parallels with the pharmacology of the
novel "abnormal-cannabidiol" receptor or a related orphan G protein-coupled
receptor, which are already known to modulate cell migration.
Endogenous lipids, phytocannabinoids, and related ligands are potent
inhibitors of human neutrophil migration, and it implicates a novel
pharmacological target distinct from cannabinoid CB(1) and CB(2) receptors;
this target is antagonized by the endogenous compound N-arachidonoyl l-serine.
Conclusion: This small, short-term,
placebo controlled trial of inhaled cannabis
demonstrated a dose dependent reduction in
diabetic peripheral neuropathy
pain in patients with treatment-refractory pain. This adds preliminary
evidence to support further research on the efficacy of the cannabinoids in
neuropathic pain.
Conclusion: The use of cannabis was associated with
beneficial effects on some Fibromyalgia symptoms.
Conclusion: Neuropathic orofacial pain (NOP) exists
in several forms including pathologies such as burning mouth syndrome (BMS),
persistent idiopathic facial pain (PIFP), trigeminal neuralgia (TN) and
postherpetic neuralgia (PHN). BMS and PIFP are classically diagnosed by
excluding other facial pain syndromes. Analgesia is one the principal
therapeutic targets of the cannabinoid system and many studies have
demonstrated the efficacy of cannabinoid compounds in the treatment of
neuropathic pain.
Conclusion: Cannabis-based medicinal extracts used
in different populations of chronic nonmalignant neuropathic pain patients may
provide effective analgesia in conditions that are refractory to other
treatments.
Conclusion: Cannabis-based medicinal extracts used
in different populations of chronic nonmalignant neuropathic pain patients may
provide effective analgesia in conditions that are refractory to other
treatments.
inflammation
IL-1 is a master regulator of
inflammation via controlling a variety of innate immune processes. Currently,
human sequence algorithm technologies suggest that the IL-1 family comprises a
total of 11 members with similar or distinct biological effects.
IL-1a,
IL-1ß, IL-1Ra, IL-18, IL-33, IL-36a, IL-36ß, IL-36α, IL-36Ra
IL-37, and IL-38 have been identified and characterized.
Among them,
IL-1a, IL-1ß, IL-18, IL-33, and IL-36 are receptor-agonistic, and IL-1Ra,
IL-36Ra, and IL-38 are receptor-antagonistic.
IL-37 is the only
anti-inflammatory
cytokine.
Although
the function of each IL-1 family member is now being investigated, IL-1 is the
most characterized among these members.
Neuroimmune networks and the brain endocannabinoid
system contribute to the maintenance of neurogenesis.
Activation of
cannabinoid receptors suppresses chronic inflammatory responses through the
attenuation of
pro-inflammatory mediators.
The endocannabinoid system directs cell
fate specification of NSCs (neural
stem cells) in the CNS (central nervous system).
The aim of our
work is to understand better the relationship between the endocannabinoid and
the IL-1ß (interleukin-1ß) associated signalling pathways and NSC
biology, in order to develop therapeutical strategies on CNS diseases that may
facilitate brain repair.
NSCs express functional CB1 and
CB2 cannabinoid receptors, DAGLa (diacylglycerol lipase a) and the
NSC markers SOX-2 and nestin. We have investigated the role of CB1
and CB2 cannabinoid receptors in the control of NSC proliferation
and in the release of immunomodulators [IL-1ß and IL-1Ra (IL-1 receptor
antagonist)] that control NSC fate decisions. Pharmacological blockade of
CB1 and/or CB2 cannabinoid receptors abolish or decrease
NSC proliferation, indicating a critical role for both CB1 and
CB2 receptors in the proliferation of NSC via IL-1 signalling
pathways. Thus the endocannabinoid system, which has neuroprotective and
immunomodulatory actions mediated by IL-1 signalling cascades in the brain,
could assist the process of proliferation and differentiation of embryonic or
adult NSCs, and this may be of therapeutic interest in the emerging field of
brain repair.
Conclusion: CB2 is involved in the
THC-induced anti-inflammation in LPS-stimulated MG-63 cells, and the
anti-inflammation may be mediated by cofilin-1.
antibiotic
Conclusion: These observations suggest that the
prenyl moiety of cannabinoids serves mainly as a modulator of lipid affinity
for the olivetol core, a per se poorly active antibacterial pharmacophore,
while their high potency definitely suggests a specific, but yet elusive,
mechanism of activity.
Conclusion: Cannabinoids have been shown to exert
anti-inflammatory activities in various in vivo and in vitro experimental
models as well as ameliorate various
inflammatory degenerative diseases.
arthritis
Conclusion: This review summarizes the promising
results that have been recently obtained in support of the therapeutic value of
cannabinoids for osteoarthritis management.
Conclusion: Our data predict that the cannabinoid
receptor system present in the synovium may be an important therapeutic target
for the treatment of pain and inflammation associated with OA and RA.
Conclusion: We discuss
the possible functions of
the endocannabinoid system in the modulation of RA, which may be a potential
target for treatment.
Conclusion: Significant analgesic effect was observed
and disease activity was significantly suppressed following Sativex
treatment.
Conclusion: CB2 offers
a molecular target for the diagnosis and treatment of osteoporosis, the most
prevalent degenerative disease in developed countries.
Osteoporosis
develops when bone mineral density and bone mass decreases.
Key nutrients for bone health at
all ages are calcium, protein and
vitamin D.
A lack of
foods that contain vital nutrients
negatively affect bone
density.
Rich sources of
vitamin D include fatty
fish, fish-liver oils and liver.
Foods fortified with
vitamin D including milk, orange juice
and cereals.
Drugs interfere vitamin D metabolism: steroids,
dilantin and phenobarbitol.
Sodium causes an increase in renal calcium
excretion.
Low potassium diets increase urinary calcium losses and high
potassium diets reduce it.
Potassium is found in several vegetables,
fruits, legumes and milk.
Accumulating evidence
suggests that cannabinoids have chondroprotective effects.
Apart from the above mentioned advantages of
cannabinoids in chronic pain, ECS modulation itself might be a useful strategy
for treating arthritis and the accompanying pain and inflammation. Although
endocannabinoids are not selective for the CB2 receptor, they have
been proven to diminish hyperalgesia in various arthritis animal models
and prevent joint damage.
Activation of cannabinoid receptor CB2
reduces inflammation. Cofilin-1 is a cytoskeleton protein, participating in the
inflammation of OA. In this study, MG-63 cells, an osteosarcoma cell-line, were
exposed to lipopolysaccharide (LPS) to mimic the inflammation of OA. We found
that THC suppressed the release of proinflammatory factors, including tumor
necrosis factor a (TNF-a), interleukin- (IL-) 1ß, IL-6, and IL-8,
decreased nuclear factor-KB (NF-KB) expression, and
inhibited the upregulation of cofilin-1 protein in the LPS-stimulated MG-63
cells. However, administration of CB2 receptor antagonist or the
CB2-siRNA, not CB1 antagonist AM251, partially abolished
the THC-induced anti-inflammatory effects above. In addition, overexpression of
cofilin-1 significantly reversed the THC-induced anti-inflammatory effects in
MG-63 cells. These results suggested that CB2 is involved in the
THC-induced anti-inflammation in LPS-stimulated MG-63 cells, and the
anti-inflammation may be mediated by cofilin-1.
The NF-KB pathway is a primary
intracellular pathway controlling the transcription of many inflammatory
enzymes.
We found that the two major cannabinoids present in marijuana,
Δ9tetrahydrocannabinol (THC) and cannabidiol (CBD), decrease
the production and release of proinflammatory cytokines, including
interleukin-1ß, interleukin-6, and interferon (IFN)ß, from
LPS-activated microglial cells. The cannabinoid anti-inflammatory action does
not seem to involve the CB1 and
CB2 cannabinoid receptors or the abn-CBD-sensitive receptors. In
addition, we found that THC and CBD act through different, although partially
overlapping, mechanisms. CBD, but not THC, reduces the activity of the
NF-KB pathway, a primary pathway regulating the expression of
proinflammatory genes. Moreover, CBD, but not THC, up-regulates the activation
of the STAT3 transcription factor, an element of homeostatic mechanism(s)
inducing anti-inflammatory events.
Both THC and CBD decrease LPS-induced
IFNß production and release. These cannabinoids exert their inhibitory
activity upstream of IFNß synthesis, e.g. at the level of the
MyD88-independent pathway that is leading to the activation of IRF-3. In
summary, our observations show that CBD and THC vary in their effects on the
anti-inflammatory pathways, including the NF-KB and
IFNß-dependent pathways.
autism
Conclusion: Our data thus suggest that neuroligin-3
is specifically required for tonic endocannabinoid signaling, raising the
possibility that alterations in endocannabinoid signaling may contribute to
autism pathophysiology.
Conclusion: These studies support a link between
cellular immune dysregulation and ASD-related behavioral deficits in a mouse
model of an autism risk factor.
dermatitis
Conclusion: The newly discovered endocannabinoid
system (ECS; comprising the endogenous lipid mediators endocannabinoids present
in virtually all tissues, their guanine nucleotide-binding protein-coupled
cannabinoid receptors, biosynthetic pathways and metabolizing enzymes) has been
implicated in multiple regulatory functions both in health and disease. It
seems that the main physiological function of the cutaneous ECS is to
constitutively control the proper and well-balanced proliferation,
differentiation and survival, as well as immune competence and/or
tolerance, of skin cells.
Pathological alterations in the activity of the fine-tuned cutaneous ECS might
promote or lead to the development of certain skin diseases.
Conclusion: This has important implications for the
future development of strategies to harness cannabinoids for the treatment of
inflammatory skin diseases.
Skin inflammatory diseases result from complex
events that include dysregulation and abnormal expression of inflammatory
mediators or their receptors in skin cells. The extract inhibited the release
of mediators of inflammation involved in wound healing and inflammatory
processes occurring in the skin. The mode of action involved the impairment of
the nuclear factor-kappa B (NF-KB) pathway since the extract
counteracted the tumor necrosis factor-alpha-induced NF-KB-driven
transcription in both skin cell lines. Cannabis extract and cannabidiol showed
different effects on the release of interleukin-8 and vascular endothelial
growth factor, which are both mediators whose genes are dependent on
NF-KB. The effect of cannabidiol on the NF-KB pathway and
metalloproteinase-9 (MMP-9) release paralleled the effect of the extract thus
making cannabidiol the major contributor to the effect observed.
Down-regulation of enzymes involved in wound healing and skin
inflammation was at least in part due to the presence of cannabidiol.
Our findings provide new insights into the potential effect of Cannabis
extracts against inflammation-based skin diseases.
Key findings: THC significantly inhibited tumor
growth of transplanted HCmel12 melanomas in a CB receptor-dependent manner in
vivo through antagonistic effects on its characteristic pro-inflammatory
microenvironment.
herpes
Conclusion: THC specifically targets viral and/or
cellular mechanisms required for replication and possibly shared by these gamma
herpesvirus, and the endocannabinoid system is possibly involved in regulating
gamma herpesvirus latency and lytic replication.
Conclusion: Small concentrations of THC were more
potent and selective against gamma herpes virus than the commonly used
antiviral drugs acyclovir, gancicyclovir and foscamet.
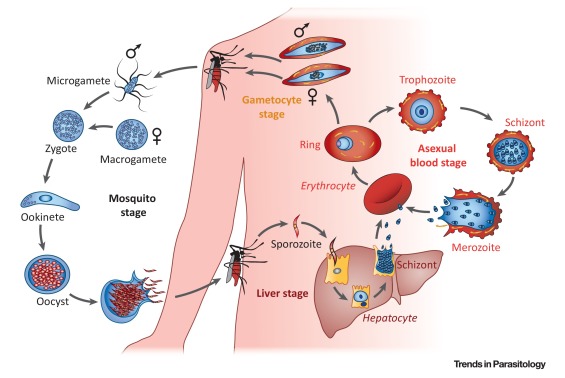
In the mosquito-human life cycle, the six species of
malaria parasites infecting humans (Plasmodium falciparum, Plasmodium
vivax, Plasmodium ovale wallickeri, Plasmodium ovale curtisi,
Plasmodium malariae, and Plasmodium knowlesi) undergo 10 or more
morphological states, replicate from single to 10,000+ cells, and vary in total
population from one to many more than 106 organisms. In the human host, only a
small number of these morphological stages lead to clinical disease and the
vast majority of all malaria-infected patients in the world produce few (if
any) symptoms in the human. Human clinical disease (e.g.,
fever, anemia, coma) is the
result of the parasite preprogrammed biology in concert with the human
pathophysiological response. Caveats and corollaries that add
variation to this host-parasite interaction
include parasite genetic
diversity of key proteins, coinfections, comorbidities, delays in
treatment, human polymorphisms, and environmental determinants.
Important strides have been made within the past
decade toward malaria elimination in many regions, and with this progress, the
feasibility of eradication is once again under discussion. If the ambitious
goal of eradication is to be achieved by 2040, all species of Plasmodium
infecting humans will need to be targeted with evidence-based and concerted
interventions. In this perspective, the potential barriers to achieving global
malaria elimination are discussed with respect to the related diversities in
host, parasite, and vector populations. We argue that control strategies need
to be reorientated from a sequential attack on each species, dominated by
Plasmodium falciparum to one that targets all species in parallel. A set of
research themes is proposed to mitigate the
potential setbacks on the pathway to a malaria-free
world.
Malaria is increasingly imported, caused by
Plasmodium vivax in settings outside sub-Saharan Africa, and clustered
in small geographical areas or clustered demographically into subpopulations,
which are often predominantly adult men, with shared social, behavioural, and
geographical risk characteristics.
Conclusion: Cerebral malaria (CM) is a severe
complication resulting from Plasmodium falciparum infection that might cause
permanent neurological
deficits. Our results indicate that CBD exhibits neuroprotective effects in
CM model and might be useful as an adjunctive therapy to prevent
neurological
symptoms following this disease.
Conclusion: Smoked cannabis was well tolerated and
effectively relieved chronic
neuropathic pain from HIV-associated sensory neuropathy. The findings are
comparable to oral drugs used for chronic neuropathic pain.
Conclusion: These results indicate that
cannabinoid-mediated inhibition of BV-2 microglial-like cell migration to Tat
is linked functionally to the CB2R. Furthermore, the results indicate that
activation of the CB2R leads to altered expression and compartmentation of the
ß-chemokine receptor CCR-3.
Conclusion: The
blood-brain barrier (BBB) is
a complex structure that is composed of cellular elements and an extracellular
matrix (ECM). HIV-1 Tat promotes transmigration of monocytes across this
barrier, a process that includes interaction with
ECM proteins. The
results indicate that cannabinoids that activate the CB2R inhibit the ECM
adhesion process. Thus, this receptor has potential to serve as a therapeutic
agent for ablating neuroinflammation associated with
HIV-elicited influx of monocytes across the
BBB.
The cannabinoid (CB) system is widespread in the
central nervous system and is crucial for controlling a range of
neurophysiological processes such as pain, appetite, and cognition. The
endogenous CB molecules, anandamide, and 2-arachidonoyl glycerol, interact with
the guanine nucleotide-binding protein coupled CB receptors, CB1 and
CB2. These receptors are also targets for the phytocannabinoids
isolated from the cannabis plant and synthetic CB receptor ligands. The CB
system is emerging as a key regulator of
neuronal cell fate and is
capable of conferring neuroprotection by the direct engagement of prosurvival
pathways and the control of neurogenesis.
Many neurological conditions
feature a neurodegenerative component
that is associated with excitotoxicity, oxidative stress, and
neuroinflammation, and certain CB
molecules have been demonstrated to inhibit these events to halt the
progression of neurodegeneration. Such properties are attractive in the
development of new strategies to treat neurodegenerative conditions of diverse
etiology, such as Alzheimer' disease, multiple sclerosis, and cerebral
ischemia.
Cannabinoids protect neurons from excitotoxic
injury. We investigated the mechanisms involved by studying
N-methyl-d-aspartate (NMDA) toxicity in cultured murine cerebrocortical neurons
in vitro and mouse cerebral cortex in vivo. Cannabinoids seem to protect
neurons against NMDA toxicity at least in part by activation of CB1R and
downstream inhibition of PKA signaling and NO generation.
Endocannabinoids act as retrograde messengers that,
by inhibiting neurotransmitter release via presynaptic CB1
cannabinoid receptors, regulate the functionality of many synapses. In
addition, the endocannabinoid system participates in the control of neuron
survival. Thus, CB1 receptor activation has been shown to protect
neurons from acute brain
injury as well as in neuroinflammatory conditions and neurodegenerative
diseases.
Cannabinoid neuroprotective activity relies on the inhibition
of glutamatergic neurotransmission and on other various mechanisms, and is
supported by the observation that the brain overproduces endocannabinoids upon
damage. Coupling of neuronal CB1 receptors to cell survival routes
such as the phosphatidylinositol 3-kinase/Akt and extracellular
signal-regulated kinase pathways may contribute to cannabinoid neuroprotective
action. These pro-survival signals occur, at least in part, by the cross-talk
between CB1 receptors and growth factor tyrosine kinase receptors.
Besides promoting neuroprotection, a role for the endocannabinoid system in the
control of neurogenesis from neural progenitors has been put forward. In
addition, activation of CB2 cannabinoid receptors on glial cells may
also participate in neuroprotection by limiting the extent of
neuroinflammation. These findings support that endocannabinoids constitute a
new family of lipid mediators that act as instructive signals in the control of
neuron survival.
The endocannabinoids (eCBs) anandamide and
2-arachidonoylglycerol are important retrograde messengers that inhibit
neurotransmitter release via presynaptic CB1 receptors. Cannabinoids
are known to modulate the cell death/survival decision of different neural cell
types. Cannabinoids protect primary neurons, astrocytes and
oligodendrocytes
from apoptosis, whereas transformed glial cells
are prone to apoptosis by cannabinoid challenge. Recent research shows that
eCBs stimulate neural progenitor proliferation and inhibit
hippocampal neurogenesis in
normal adult brain. Cannabinoids inhibit cortical neuron differentiation
and promote glial differentiation. Cannabinoids also regulate neuritogenesis,
axonal growth and synaptogenesis. These new observations support that eCBs
constitute a new family of lipid signaling cues responsible for the regulation
of neural progenitor proliferation and differentiation, acting as instructive
proliferative signals through the CB1 receptor.
Cannabinoid receptor agonists act presynaptically to
inhibit glutamate release. The effect
of prolonged drug exposure on the neuroprotection afforded by cannabinoid
receptor agonists was also studied. Desensitization of CB(1) receptors
diminishes the neuroprotective effects of cannabinoids. This study demonstrates
the importance of agonist efficacy and the duration of treatment on the
neuroprotective effects of cannabinoids.
In summary, we have shown that in an in vivo model
of neurodegeneration Δ9-THC reduces neuronal damage via a
CB1-receptor-mediated mechanism. This holds in both the acute and
late phase after induction of excitotoxicity. Δ9-THC inhibits
astrogliosis via a non-CB1-receptor-controlled mechanism. The
results strengthen the concept that the endogenous cannabinoid system may serve
to establish a defense system for the brain. This system may be functional in
several neurodegenerative diseases in which excitotoxicity is thought to play a
role, such as amyotrophic lateral
sclerosis, Huntington' and Parkinson'
diseases, and also in acute
neuronal damage as found in stroke and
traumatic brain injury.
It is conceivable that the endogenous cannabinoid system can be exploited for
therapeutic interventions in these types of primarily incurable diseases.
The protective effect of cannabidiol (CBD), the
non-psychoactive element of Cannabis sativa, against neuronal toxicity induced
by cadmium chloride (CdCl2 10 µM) was investigated in a retinoic acid
(RA)-differentiated SH-SY5Y neuroblastoma cell line. These data showed that
Cd-induced neuronal injury was
ameliorated by CBD treatment and it was concluded that CBD may represent a
potential option to protect neuronal cells from the detrimental effects of Cd
toxicity. |